* These authors contributed equally
Introduction
Causes of death and, in particular, deaths due to infection have not been widely studied in randomised trials in chronic lymphocytic leukaemia (CLL). With long-term follow-up (median 13 years), we were able to examine the cause of death in 600/777 patients in the LRF CLL4 trial. Blood samples taken at randomization from 499 patients were available, allowing us to examine the relationship between deaths due to infection and a large panel of genes which are commonly mutated in CLL. Several gene mutations have been linked to earlier death in the LRF CLL4 trial, including mutations of TP53, NOTCH1, SF3B1, EGR2 and MAPK-ERK (Gonzalez et al, J Clin Oncol 2011; 29:2223-9; Oscier et al, Blood 2013; 121:468-75; Young et al, Leukemia 2017; 31:1547-54; Blakemore et al, Leukemia 2020; 34:1760-4). In this study we aimed to identify gene mutations which were specifically associated with death due to infection.
Methods
In LRF CLL4 patients were randomized between 1999-2004 to receive chlorambucil or fludarabine, with or without cyclophosphamide. Follow-up continued until September 2016. Causes of death were assessed centrally by the principal investigator.
Results
In the LRF CLL4 trial 614 of 777 patients (79%) died before the end of follow-up. The cause of death was known in 600 patients. Deaths tended to be multifactorial, but infection was a cause of death in 258 patients (43%). Fatal infections were pneumonia (67%), and/or sepsis (38%) and/or opportunistic infections such as aspergillus (11%).
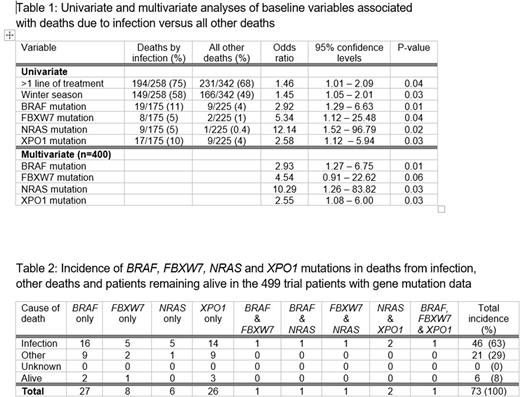
Patients who died of infection were more likely than those who died of other causes to have received more than one line of treatment and to have died in the winter months (Table 1). Mutations of BRAF, FBXW7, NRAS and XPO1 were significantly associated with death due to infection versus other deaths. However, with multiple hypothesis testing, NRAS was the only genetic mutation to survive a false discovery rate (FDR) q-value = 0.05 (odds ratio: 17, P = 0.0004). No other significant differences were found between patients who died of infection versus those whose death did not have an infectious cause. In particular, the rate of deaths due to infection was not influenced by other demographic or laboratory factors, nor by the randomised treatment, the response to treatment, or the size/experience of the CLL treatment centre. In multivariate analysis the factors most significantly associated with death from infection versus all other deaths were mutations of the BRAF, FBXW7, NRAS and XPO1 genes (Table 1).
Of the 499 patients in the trial for whom gene mutation data were available, 73 (15%) carried one or more of the four gene mutations BRAF (6%), FBXW7 (2%), NRAS (2%) and XPO1 (6%) (Table 2). Only six of these 73 remained alive. Death was caused by infection in 46/67 assessable patients (69%) who had a mutation of one or more of these four genes versus only 129/333 patients (39%) without any of these mutations (odds ratio: 3.46 [95% C.I. 1.98-6.07] P<0.0001).
In order to test the robustness of our results, the same analysis was repeated in the full trial, comparing the patients who died of infection with all the other trial patients, including those who remained alive. The presence of one or more of the four gene mutations BRAF, FBXW7, NRAS and XPO1 was the most significant predictor of death from infection in univariate analysis in this larger dataset (odds ratio: 3.92 [95% C.I. 2.34-6.59] P<0.0001). Patients who died of infection lost on average 2 years 4 months of life compared with the median overall survival of all the other trial patients (6 years 11 months, log-rank P<0.0001).
Conclusion
Patients in LRF CLL4 were at some risk of death due to infection, irrespective of their demographic characteristics, disease stage and treatment history. Nevertheless, those who had received more lines of treatment were particularly at risk, as were those who carried a BRAF, FBXW7, NRAS or XPO1 mutation. A meta-analysis of datasets from other trials could be important to assess the validity of the link between these gene mutations and deaths from infections in patients with CLL and possibly other leukaemias and lymphomas. Careful management of infection risk, together with prophylaxis against infection, may be important in patients who carry one or more of these mutations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal